Enabling Precision in Immuno-Oncology Research
Join us in Philadelphia, August 11–13, to explore how GXP-Storage supports cutting-edge cancer research with compliant, scalable, and audit-ready biostorage solutions.
Whether you're managing biospecimens for translational studies, coordinating multi-site clinical trials, or navigating GCP/GMP requirements, our infrastructure is designed to meet the rigorous demands of oncology research.
Visit GXP-Storage® at Booth #10 to explore how integrated lifecycle management transforms compliance and protects therapeutic integrity.

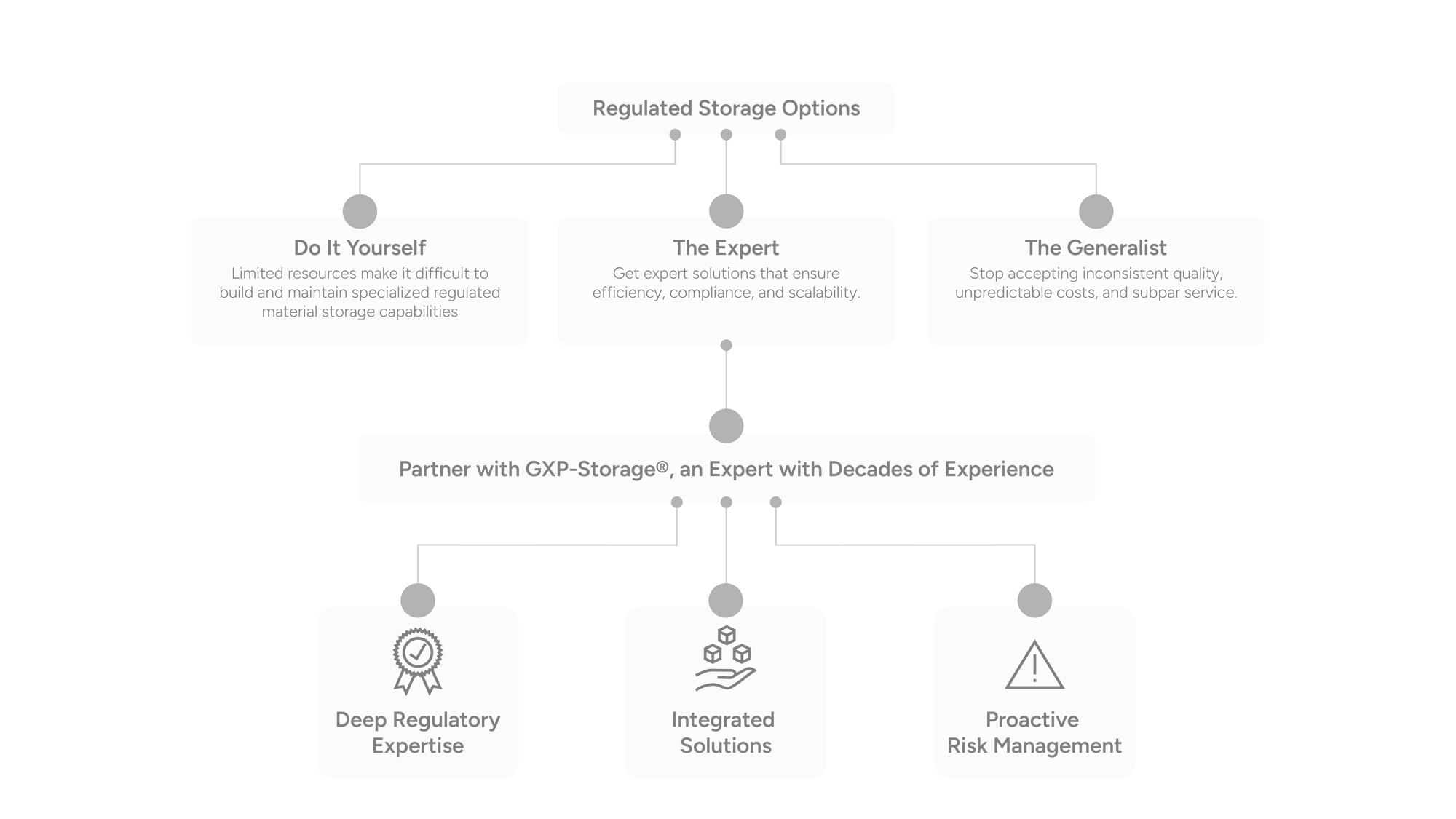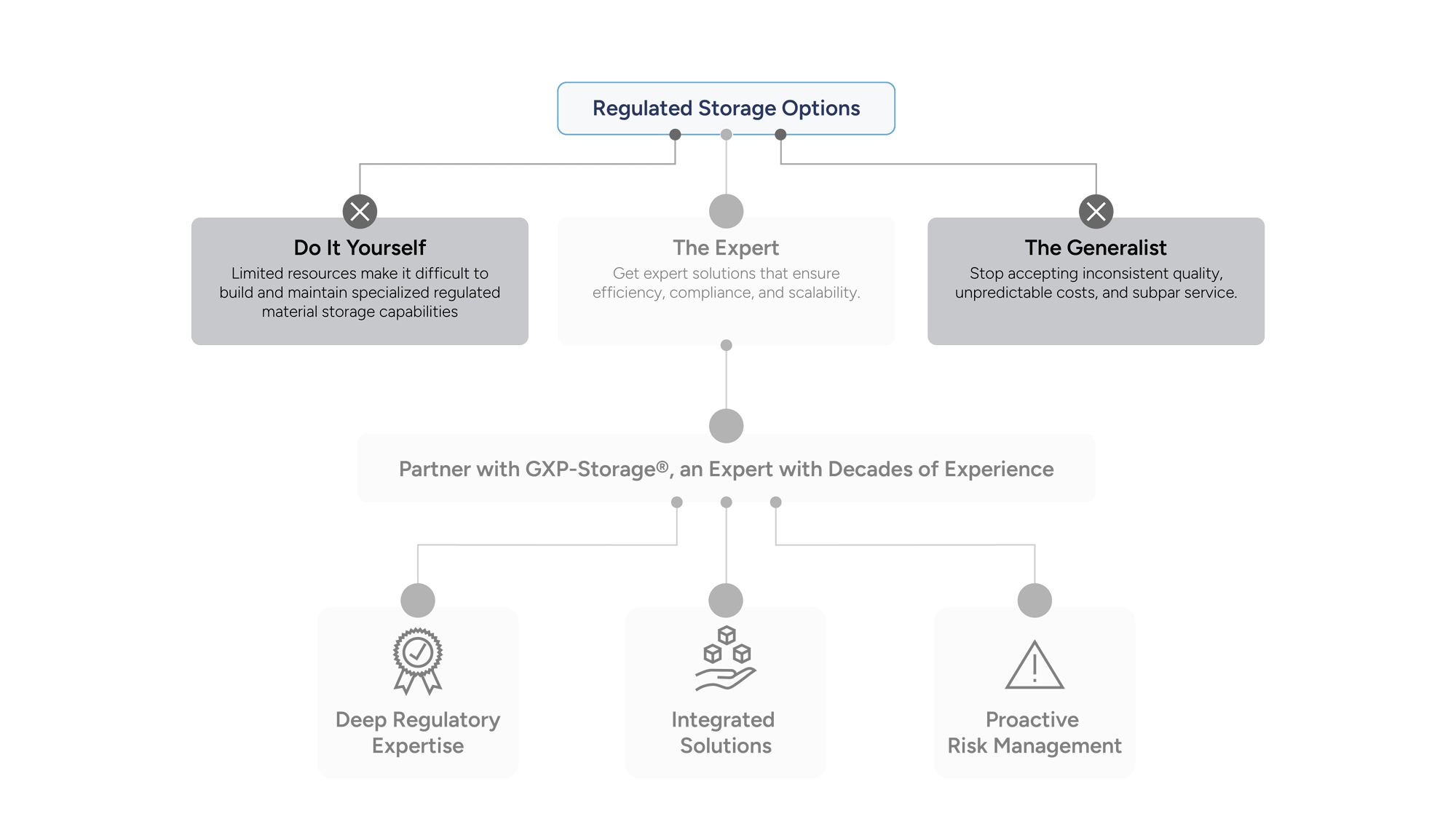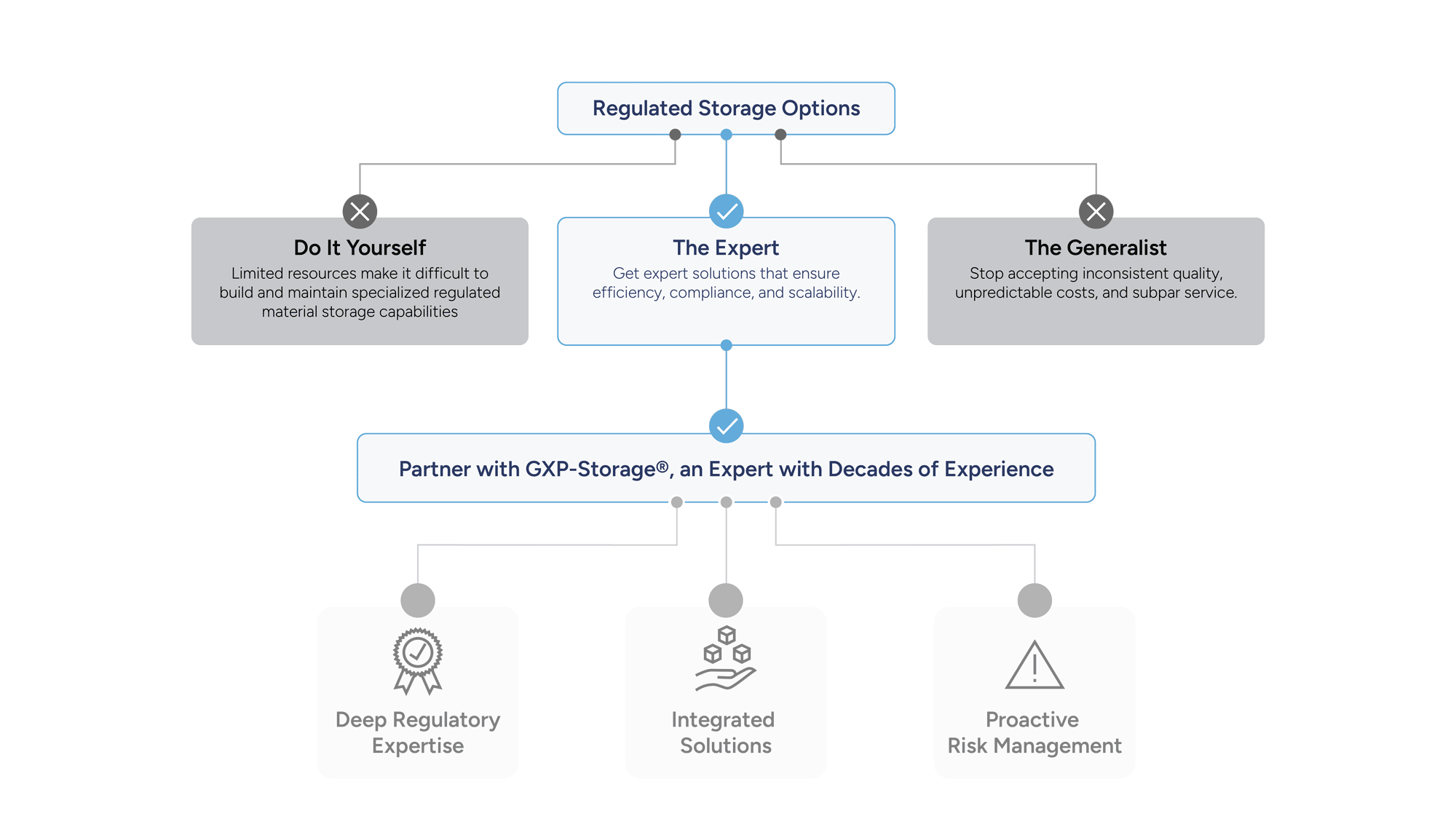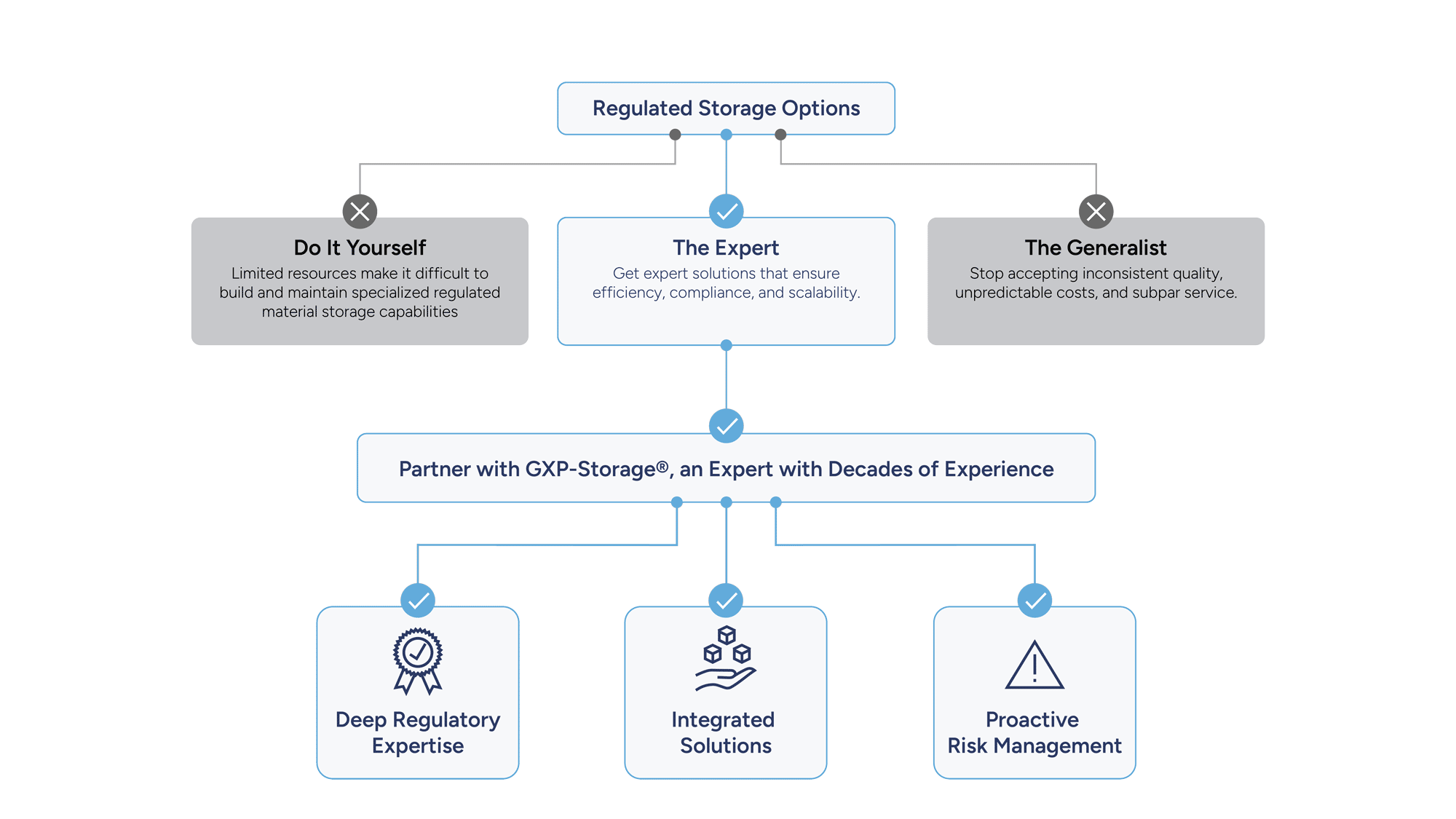
Why Researchers Choose GXP-Storage
Researchers in immuno-oncology rely on precision, compliance, and scalability. GXP-Storage delivers purpose-built solutions that meet the demands of modern scientific workflows, ensuring every sample and process is protected, traceable, and ready for what’s next.
Built for Scientific Rigor: Maintain sample integrity with validated temperature control, full chain-of-custody, and real-time monitoring.
Accelerate Clinical Readiness: With the power of our GXP-Guardian platform at your fingertips, you gain centralized visibility of your material across multiple sites, borders, and vendors, enabling faster, seamless retrieval from discovery to late-stage development
Compliance Without Compromise: Align with FDA, EMA, and ICH guidelines while scaling your research operations.
Our systems are designed for inspection readiness and clinical scalability, allowing you to focus on patient outcomes rather than vendor management.
Book a Meeting at Booth #10
What You'll Learn
Discover how GXP-Storage® is redefining material management for research organizations and healthcare institutions. Each of the following topics will be featured at our booth, click in to explore how we’re solving the industry’s most pressing challenges:
GXP-Guardian®: Real-Time Oversight for Regulated Research Environments
GXP-Guardian® is a validated, AI-enabled platform purpose-built for managing the lifecycle of regulated research materials. It delivers real-time visibility across all storage environments, on-site or third-party, while automating compliance documentation, audit trails, and predictive analytics. Designed for research teams navigating GCP/GMP frameworks, GXP-Guardian® reduces manual oversight and helps ensure inspection readiness without compromising scientific agility.
Unify Research Storage Across Sites and Studies
Fragmented storage introduces risk, delays, data gaps, and compliance exposure. GXP-Storage® centralizes regulated research materials into a unified, access-controlled environment. Whether you're managing biospecimens, investigational products, or assay samples, our platform ensures consistent standards, seamless transitions, and full chain-of-custody documentation across departments, collaborators, and trial sites.
Modernize Research Material Workflows with Scalable Infrastructure
GXP-Storage® empowers research institutions and clinical trial networks with scalable, purpose-built storage solutions that integrate seamlessly with lab operations and investigational product workflows. From ambient to cryogenic conditions, our infrastructure supports faster access, tighter coordination, and full regulatory alignment across every phase of your study.
Streamline Audit Readiness and Safeguard Research Continuity
Audit preparation shouldn’t stall your science. GXP-Storage® enables continuous inspection readiness with automated reporting, real-time alerts, and centralized documentation. By integrating storage, monitoring, and compliance into a single lifecycle platform, we help reduce regulatory risk and prevent disruptions across your research supply chain.
What our customers say
“GXP-Storage has been responsive and hands-on. Their systems and teams are reliable, exactly what we need to maintain confidence and compliance.”
"We were thoroughly impressed by the level of documentation, chain-of-custody controls, and readiness for regulatory inspection. It’s rare to find a storage partner so aligned with our GXP standards."
"Coordinating sample logistics across multiple trial sites and vendors can be a nightmare, but GXP Storage streamlined it all. Their team is incredibly responsive, and the GXP-Guardian platform gives us real-time tracking and centralized control we didn’t have before. It’s transformed how we manage clinical supply chains."
Let's talk at Booth #10.
Explore how we help research organizations and healthcare institutions simplify storage, safeguard patient care, and improve compliance.
Need Clarification?
What makes GXP-Storage different from traditional storage providers?
GXP-Storage® goes beyond basic warehousing by offering integrated lifecycle management for regulated healthcare and life sciences materials. Our purpose-built facilities and validated systems ensure compliance, visibility, and scalability from discovery through distribution, so you can focus on patient outcomes, not vendor coordination.
What is GXP-Guardian® and how does it help my organization?
GXP-Guardian® is our proprietary, validated platform for regulated material lifecycle management. It provides real-time visibility across all storage environments, including on-site, partner, and GXP-Storage facilities, while automating compliance documentation, chain-of-custody tracking, and audit readiness.
How does GXP-Storage support compliance with healthcare regulations?
Our systems are built to meet and exceed requirements from the FDA, DEA, HIPAA, and Joint Commission. We maintain ISO 9001:2015 certification and adhere to GLP, GCP, and cGMP standards. Every material movement is tracked and documented to ensure inspection readiness and regulatory confidence.
Can GXP-Storage integrate with our specialty pharmacy or hospital systems?
Yes. GXP-Storage® is designed for seamless integration with specialty pharmacies, hospital networks, and clinical workflows. Our technology and service model support real-time inventory visibility, temperature monitoring, and secure access across departments and locations.
What types of materials can GXP-Storage handle?
We manage a wide range of regulated materials including investigational drugs, diagnostic samples, surgical pathology, biologics, and clinical documentation. Our facilities support controlled ambient to cryogenic conditions, ensuring the integrity of even the most sensitive materials.
How do I book a meeting or schedule a discovery call?
You can use the CTA buttons on this page to book a meeting with our team at Booth #10 or schedule a discovery call at your convenience. We’ll walk you through how GXP-Storage® can support your organization’s material management goals.